The historically relevant D/L system
relates chiral molecules to the D and
L enantiomers of glyceraldehyde. The stereodescriptors
D and L are accepted in CurlySMILES
notations for molecules with one asymmetric atom:
|
||||||||
|
||||||||
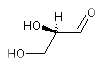
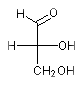
The systematic name of D-glyceraldehyde is
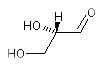
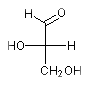
(R)-2,3-dihydroxypropanal: OCC{D}(O)C=O and OCC{R}(O)C=O encode the same enatiomer. The systematic name of L-glyceraldehyde is
(S)-2,3-dihydroxypropanal: OCC{L}(O)C=O and OCC{S}(O)C=O encode the same enatiomer. Another example is L-cysteine with the systematic name
(R)-2-amino-3-sulfanylpropanoic acid: O=C(O)C{L}(N)CS and O=C(O)C{R}(N)CS encode the same enatiomer. Molecules with more than one stereogenic center should always be encoded by using the R and S stereodescriptors, illustrated for (2R,3R)-2,3,4-trihydroxybutanal, commonly known as D-erythrose:O=CC{R}(O)C{R}(O)CO |
||||||||
Tweet
_
__
__
 __
__
__ ___ 
|
References
| [1] |
B. Testa:
Principles of organic stereochemistry. Marcel Dekker, New York and Basel, 1979. |
| [2] |
D. Hellwinkel:
Systematic Nomenclature of Organic Chemistry. Springer-Verlag, Berlin und Heidelberg, Germany, 2001. |
| [3] | A. Drefahl: CurlySMILES: a chemical language to customize and annotate encodings of molecular and nanodevice structures. J. Cheminf. 2011, 3:1; doi: 10.1186/1758-2946-3-1 . |
| Encoding of stereoisomers |
|
R and S
enantiomers E and Z stereoisomers |

Custom Search
|