|
E/Z isomers
(cis/trans isomers)
are encoded with CurlySMILES using the
curly codes
{E} and {Z}, which are
applied to pairs of atoms that are connected by a double bond.
The anchor atom for a curly code
of this type is always that atom of the pair that comes second
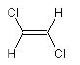
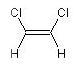
in the overall notation—reading from left to right. The
following example illustrates this for the two
1,2-dichloroethene stereoisomers:
|
|
|
|
ClC=C{E}Cl
|
ClC=C{Z}Cl
|
|
(E)-1,2-dichloroethene
|
(Z)-1,2-dichloroethene
|
|
|
Notice that the stereodescriptors E and Z
are assigned according to the IUPAC sequencing rules for
substituents and not according to
cis or trans
position of substituents of the same kind (see, for example,
page 74 in [1] or page 187 in [2]). For example, the
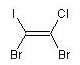
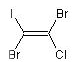
1,2-dibromo-1-chloro-2-iodoethene
molecule with cis positioning of the two bromo atoms
is the E isomer and the molecule with trans
Br-atoms is the Z isomer:
|
|
|
|
BrC(I)=C{E}(Br)Cl
|
BrC(I)=C{Z}(Br)Cl
|
|
(E)-1,2-dibromo-1-chloro-2-iodoethene
|
(Z)-1,2-dibromo-1-chloro-2-iodoethene
|
|
|
Stereoisomers, in which one or both double-bond atoms are
heteroatoms, are similarly designated via IUPAC rules
and accordingly encoded with CurlySMILES, as shown for
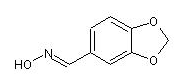
piperonal (E)-oxime
(traditionally named piperonal syn-oxime) and
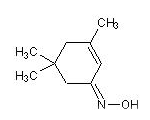
3,5,5-trimethylcyclohex-2-ene
(Z)-oxime:
|
|
|
|
ON=C{E}c1cc2OCOc2c1
|
C1=C(C)CC(C)(C)CC1=N{Z}O
|
|
piperonal (E)-oxime
|
3,5,5-trimethylcyclohex-2-enone (Z)-oxime
|
|
_
__
__
 __
__
__
Share on Tumblr
___

|
 __
__







