A homopolymer is derived from a single monomer species. Formally,
a homopolymer is obtained by deriving a bivalent group
from the monomer, constituting a structural repeat unit
(SRU). For example, from the monomer molecule 3-hexylthiophene, the
SRU is derived by removing the two H-atoms at position
2 and 5 of the thiophene ring. The following notation encodes the
3-hexylthiophene-derived SRU:
c{-}(c1)cc(CCCCCC)c1{-}
The ring positions 2 and 5 correspond to the node positions 1 and 11 in the string, respectively.
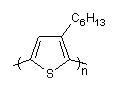
The backbone of poly(3-hexylthiophene), PH3T, is formally constructed by connecting
the C-atom at node position 1 to the C-atom at node position 11, n times:
|
|
|
c{-}(s1)cc(CCCCCC)c1{+n}
|
|
Poly(3-hexylthiophene) backbone
|
|
The PH3T polymer with terminating H atoms is encoded as:
[H]c{-}(s1)cc(CCCCCC)c1{+n}[H]
The atoms belonging to the SRU are identified by back-scanning from the
{+n} annotated node to the first
{-} annotated node at the same depth level.
If nodes that belong to the SRU are located outside this span— or, if
nodes inside this span are not part of the SRU—then they need to be indicated.
This is done by using the
annotation dictionary keys
inc and exc to include and exclude atoms.
The following string, an alternative notation to the one given above, illustrates
SRU inclusion and exclusion of atoms:
s1c{-}([H])cc(CCCCCC)c1{+ninc=1;exc=3}[H]
The value of an inc or exc key is a single integer or
a comma-separated list of integers specifying atoms by their node position within
the notation. In the given example, the S-atom at position 1 is included and
the H-atom at position 3 is excluded.
|
_
 __
__
|

